Data Requests
Access to Data and Types of Research Study
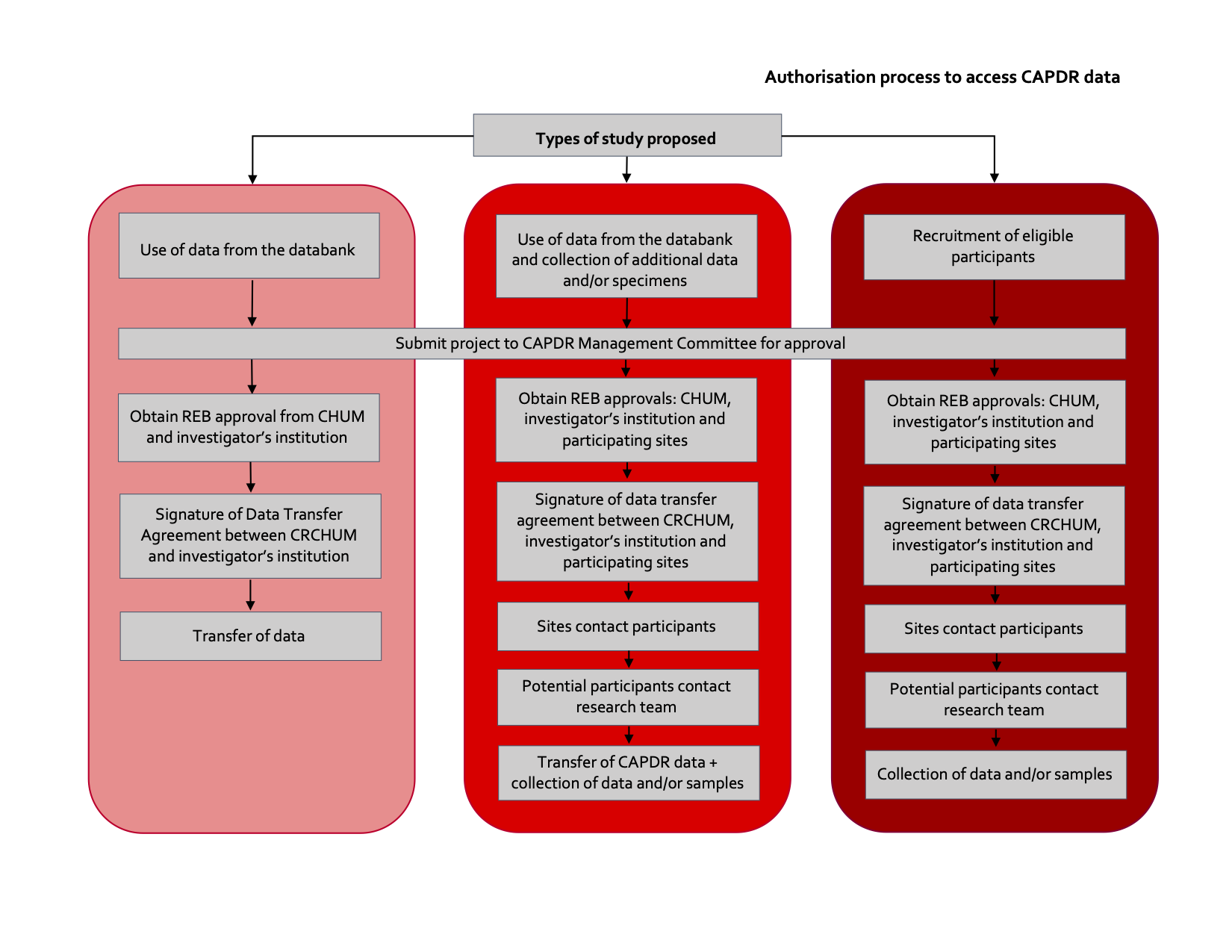
CAPDR data is made available to CPN members and external collaborators in various disciplines who wish to develop scientific knowledge in the field of chronic pain. The CAPDR database is made available to Canadian investigators and investigators from abroad who are conducting research and who are linked with public or private organizations. Use of the CAPDR database is subject to conditions that, on the one hand, make it possible to respect the consent provided by participants and, on the other hand, allow this information to be used as efficiently as possible. The data attribution process is described under Figure 1. Each study must first undergo a scientific and feasibility evaluation by the CAPDR Management Committee (CAPDR MC). Should the CAPDR MC lack the expertise to evaluate a proposed project, an additional opinion on the project will be obtained from an external expert.
The structure of the CAPDR allows the conduct of three different types of research study:
- Research studies using CAPDR database data;
- Research studies using CAPDR database data, in combination with other variables or biological specimens;
- Research studies aiming to recruit CAPDR participants for new research studies according to specific selection criteria.
Application to Access Data
Applicants who wish to access the Database must submit the following documents:
- Completed Data Access Request Form
- Study protocol
- Study budget
- Institutional ethics approval
- Proof of funding (if applicable)
- CV of the Principal Applicant
In their application, applicants must indicate the objectives of the research project, scientific bases, methods, funding, expected benefits, foreseeable risks, measures aimed at protecting confidentiality, duration, investigators involved in the project including their affiliation, and type of data needed.
Following the evaluation of the project, the CAPDR-MC makes a well-founded decision in writing. Applicants authorized to access CAPDR database data will receive a a Data Access Authorisation letter. The CAPDR-MC may impose conditions on its decision. It will also consider requests from applicants who are applying for funding and are requesting a letter of support from the CAPDR-MC.
Access to the CAPDR database data will be granted for a finite period of time. Renewal of CAPDR database data access approval will be required to extend use of data after that period.
Evaluation Criteria of Requests
Any application to use data from the CAPDR database must meet the following criteria:
- The project is aimed at developing knowledge about chronic pain;
- The project must be feasible and scientifically valid;
- The investigator will provide sufficient funding in the event additional data/specimen collection is required on site;
- The investigator and the investigator’s team, if applicable, have the required knowledge, qualifications and resources to conduct the project;
- The planned use of the data is in accordance with the consent provided by participants;
- The project provides measures to adequately protect the data;
- The project is acceptable in terms of research ethics.
Transfer of Data for Research Studies
The CHUM REB must approve any new research project before CAPDR data can be transferred for research purposes. Should the project require contacting participants in order to obtain an additional consent (e.g. additional collection of data and/or samples, participation in new research projects), REB approval must be obtained from all sites where participants are contacted, and from the investigator’s institution.
Following approval of the project by the REBs, a Data Transfer Agreement will be concluded with each authorized user accessing data for research purposes. This agreement will include a clause stating that a limited number of people can access the data, and the complete list of authorized people will have to be provided to the CAPDR Registry Coordinator at the CHUM. Use of the CAPDR database data cannot be for any other purpose than the one approved by the CAPDR-MC as part of the Data Authorization Access letter. In addition, authorized users will have to provide their procedures for storage and destruction of data/results, which will be annexed to the Data Transfer Agreement.
Progress and Results of Research Studies
Authorized users who have been granted access and obtained CAPDR data for research purposes will have to send to the CAPDR-MC an annual progression summary for the duration of their study, and a final report. The latter will summarize research findings, resulting benefits to the public, and a list of publications generated using CAPDR data. In addition, applicants will be asked if they have any suggestions or comments regarding data access procedures.
Return of Research Results
No return of results or research findings are expected when CAPDR data is used for research purposes. Exceptionally, a clause to this effect may be integrated to the Data Transfer Agreement if an authorized user and the CAPDR-MC have agreed to it.
Publication of Results
Publications and presentations about findings from research projects that used CAPDR data must respect certain rules. They must respect the Tri-Agency Framework: Responsible Conduct of Research (2016) and Tri-Agency Open Access Policy on Publications (2015) of the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Social Sciences and Humanities Research Council of Canada (SSHRC).
Authorized users must agree beforehand to respect the requirements described below.
At a minimum, publications of results based on CAPDR data must respect the following terms:
- Including as authors, with their consent, all those and only those who have made a substantial contribution to, and who accept responsibility for the content of the publication or document. The substantial contribution may be conceptual or material
- Acknowledging appropriately all those and only those who have contributed to research, including funders and sponsors
See also Section Acknowledgements.
Authorship
Registry Working Group members, CAPDR-MC members, and Local Investigators will be listed by name if and only if their contributions to a given article or abstract justify it. In addition, external collaborators may be included as listed authors if their contributions justify it. When the number of authors is limited, investigators may be listed collectively as ‘CAPDR Team’.
Acknowledgements
For all publications that used data from the CAPDR database, contribution of the CAPDR, the CPN and its sponsors must be acknowledged as follow:
“(Part of the) Data used for this research was provided by the Canadian Adult Pain Data Registry of the Chronic Pain Network, which is(was) funded by the Canadian Institutes of Health Research as part of its Strategy for Patient-Oriented Research Program.”
Intellectual Property
The CAPDR is not for profit. Should any inventions, discoveries, new uses, processes, or compounds (the “Invention(s)”) arise directly out of a study which used data from the CAPDR, they shall be owned by the inventing party in accordance with the institutional policies of the inventing Party.

